Cadmium is a chemical element with the symbol “Cd” and atomic number 48. It is a soft, bluish-white metal that belongs to the transition metals group in the periodic table. Discovered in 1817 by German chemist Friedrich Stromeyer, cadmium has since found various industrial applications despite its known toxicity.



In its pure form, cadmium is relatively rare in the Earth’s crust, occurring at low concentrations. However, it is often found as a minor component in zinc, lead, and copper ores, from which it is extracted as a byproduct during the refining process. Due to its ability to form alloys with other metals and its resistance to corrosion, cadmium has historically been used in various industries.
One of the significant historical uses of cadmium was in pigments, particularly for creating bright yellow, orange, and red colors in paints and ceramics. However, due to its high toxicity and potential health risks, its use in this regard has decreased significantly in recent times.
In modern industries, cadmium is primarily employed in the production of nickel-cadmium (NiCd) batteries. These batteries were widely used in portable electronics and other applications due to their high energy density and long life. However, in response to environmental concerns and the hazardous nature of cadmium, other battery technologies, such as lithium-ion batteries, have become more prevalent.
Aside from batteries, cadmium has applications in electroplating, where it is used to provide a protective coating on various metals. It is also used in some types of semiconductors and photovoltaic cells for solar energy conversion.
One of the most critical aspects of cadmium is its toxicity. Cadmium and its compounds are highly toxic to humans and many other living organisms. Prolonged exposure to cadmium can lead to severe health problems, particularly affecting the kidneys and bones. Inhalation of cadmium fumes or ingestion of cadmium-contaminated food or water are common routes of exposure for humans.
Due to its hazardous nature, many countries have implemented strict regulations on the use and disposal of cadmium and cadmium-containing products to protect human health and the environment.
In conclusion, cadmium is a unique element with both beneficial industrial properties and significant health and environmental risks. Understanding its properties, sources, and potential impacts is essential for ensuring responsible usage and handling to safeguard human health and preserve the environment.
Chemical Properties of Cadmium

Cadmium (Cd) is a transition metal with several distinct chemical properties. Here are some key characteristics of cadmium from a chemical standpoint:
- Atomic Number and Mass: Cadmium has an atomic number of 48 and an atomic mass of approximately 112.41 g/mol.
- Electronic Configuration: The electron configuration of cadmium is [Kr] 4d^10 5s^2, meaning it has two electrons in its outermost shell.
- Valence Electrons: Cadmium has two valence electrons in its outermost shell, located in the 5s orbital.
- Oxidation States: The most common oxidation state of cadmium is +2, where it loses its two valence electrons to form Cd^2+ ions. Cadmium can also exist in other oxidation states, such as +1 and +3, but they are less common.
- Reactivity: Cadmium is a relatively reactive metal, but it is not as reactive as alkali metals or some other transition metals. It readily forms compounds with other elements.
- Corrosion Resistance: One of the notable properties of cadmium is its excellent resistance to corrosion. This property makes it valuable in various industrial applications, particularly for plating metals to protect them from corrosion.
- Formation of Complexes: Cadmium can form stable complexes with various ligands due to its ability to donate its two valence electrons. These complexes have applications in analytical chemistry and industrial processes.
- Reaction with Oxygen: Cadmium readily reacts with oxygen in the air, forming a thin oxide layer on its surface. This oxide layer provides some protection against further corrosion.
- Solubility: Some cadmium compounds, particularly salts, are soluble in water. The solubility depends on the specific compound and conditions.
- Toxicity: Cadmium and its compounds are highly toxic to humans and other living organisms. The toxicity is primarily attributed to its ability to interfere with various biological processes and its tendency to accumulate in the body over time.
Due to its toxicity, cadmium usage and exposure are heavily regulated to protect human health and the environment. Industries that work with cadmium or cadmium-containing materials must follow strict safety guidelines to minimize the risk of exposure to this hazardous metal.
Occurrence and Sources of Cadmium

Cadmium is relatively rare in the Earth’s crust, occurring at low concentrations. It is not found as a native metal but is commonly found as a minor component in various minerals and ores. Here are the main occurrences and sources of cadmium:
- Zinc, Lead, and Copper Ores: The primary source of cadmium is as a byproduct during the extraction and refining of zinc, lead, and copper ores. Cadmium is often present in these ores as impurities. When these metals are extracted, cadmium is also extracted and separated during the process.
- Sphalerite (Zinc Blende): The most significant mineral source of cadmium is sphalerite, which is a zinc sulfide mineral (ZnS). Sphalerite can contain varying amounts of cadmium as an impurity, and when zinc is extracted from sphalerite, cadmium is also obtained.
- Greenockite: Greenockite is a rare mineral that is a direct source of cadmium. It is a cadmium sulfide mineral (CdS) and forms as a secondary mineral in certain hydrothermal ore deposits.
- Cadmium-Bearing Minerals: Cadmium can also be found in trace amounts in other minerals, including wurtzite (another zinc sulfide), as well as in certain phosphate minerals and iron-nickel-cobalt ores.
- Phosphate Fertilizers: In some cases, cadmium can be present in phosphate fertilizers. The presence of cadmium in fertilizers can lead to its accumulation in soil, potentially impacting crops and food.
- Industrial Emissions: Cadmium is released into the environment through industrial processes such as mining, smelting, and the burning of fossil fuels. These activities can release cadmium-containing dust and fumes, leading to potential environmental contamination.
- Waste and Landfills: Improper disposal of cadmium-containing products, such as batteries and electronic waste, can contribute to cadmium pollution in soil and water bodies.
- Natural Weathering: Cadmium can also be released into the environment through natural processes, including the weathering of rocks and minerals that contain cadmium.
Once released into the environment, cadmium can persist for long periods and can be transported through air and water. It can contaminate soil, water bodies, and the food chain, posing significant risks to human health and the ecosystem. Due to its toxic nature, the management and control of cadmium sources and its safe disposal are crucial to prevent adverse effects on both human health and the environment.
Cadmium Minerals




Cadmium is commonly found as an impurity in various minerals rather than occurring as a primary mineral itself. The most important cadmium minerals are typically associated with zinc, lead, and copper ores. Here are some of the main cadmium minerals:
- Sphalerite (Zinc Blende) – Chemical Formula: (Zn,Fe)S Sphalerite is the most significant mineral source of cadmium. It is a zinc sulfide mineral and commonly contains small amounts of cadmium as an impurity. When zinc is extracted from sphalerite during the refining process, cadmium is also obtained as a byproduct.
- Greenockite – Chemical Formula: CdS Greenockite is a rare mineral and is the only direct mineral source of cadmium. It is a cadmium sulfide mineral and forms as a secondary mineral in certain hydrothermal ore deposits. Due to its bright yellow color, greenockite is sometimes used as a minor ore of cadmium and as a collector’s mineral.
- Wurtzite – Chemical Formula: (Zn,Fe)S Wurtzite is another zinc sulfide mineral that can contain cadmium as an impurity, similar to sphalerite. It is less common than sphalerite but can still be a source of cadmium during zinc extraction.
- Hawleyite – Chemical Formula: CdS Hawleyite is a rare cadmium sulfide mineral that can form as a secondary mineral in low-temperature hydrothermal environments. It is usually found in association with other cadmium minerals and zinc ores.
- Cadmite – Chemical Formula: CdCO3 Cadmite is a cadmium carbonate mineral, but it is relatively rare. It can be found as a secondary mineral in the oxidation zones of some cadmium-rich ore deposits.
- Monteponite – Chemical Formula: CdO Monteponite is a rare cadmium oxide mineral, and its occurrence is closely associated with other cadmium minerals and zinc ores.
It’s important to note that cadmium minerals are not typically mined specifically for their cadmium content. Instead, cadmium is mainly obtained as a byproduct during the extraction and refining of zinc, lead, and copper ores. The concentration of cadmium in these minerals can vary, and the specific minerals containing cadmium depend on the geological conditions of the ore deposit.
Mining and Extraction of Cadmium Ore
Mining and extraction of cadmium ore involve several steps and processes to obtain cadmium as a valuable byproduct. The primary source of cadmium is as an impurity in zinc, lead, and copper ores. Here is an overview of the typical mining and extraction process for cadmium:
- Exploration and Site Selection: The first step is to identify potential ore deposits that may contain cadmium. Geologists and mining companies use various exploration techniques, such as geophysical surveys, drilling, and geological mapping, to assess the presence and extent of cadmium-bearing minerals in the ore deposits.
- Mine Development: Once a suitable ore deposit is identified, the site goes through the mine development phase. This involves constructing access roads, developing underground tunnels or open-pit mines, and establishing infrastructure for mining operations.
- Ore Extraction: Cadmium ore is typically extracted alongside zinc, lead, or copper ores. Depending on the specific ore deposit, different mining methods may be used, such as underground mining for deep ore deposits or open-pit mining for shallow ore bodies.
- Ore Crushing and Grinding: The extracted ore is then crushed and ground into a fine powder to increase its surface area for subsequent processing. This step allows for the efficient release of the valuable minerals, including cadmium.
- Flotation: The crushed and ground ore undergoes a process called flotation. In this process, chemicals and reagents are added to the ore slurry to create conditions where valuable minerals (such as zinc, lead, and copper sulfides) are selectively separated from other non-valuable minerals.
- Concentration: The flotation process results in a concentrate that contains various metals, including cadmium. The concentrate is further processed to increase the cadmium content and remove impurities.
- Roasting: The concentrate may undergo roasting, a high-temperature process where it is heated in the presence of air or oxygen. Roasting converts cadmium sulfide minerals (like greenockite) into cadmium oxide (CdO).
- Reduction and Smelting: The roasted concentrate is mixed with carbon and heated in a furnace to reduce the cadmium oxide to metallic cadmium. The cadmium vaporizes and is then condensed to form cadmium metal.
- Refining: The obtained cadmium metal may undergo further refining to remove any remaining impurities and ensure its purity.
- Byproduct Recovery: The primary objective of mining cadmium ore is to obtain other valuable metals like zinc, lead, or copper. Cadmium is considered a valuable byproduct of this process, and its extraction is economically viable because of its industrial applications.
- Environmental Considerations: Throughout the entire mining and extraction process, measures are taken to mitigate environmental impacts, such as dust and water pollution. Responsible mining practices include reclamation and rehabilitation of the mining site to minimize long-term environmental effects.
Due to the toxic nature of cadmium and its potential impact on human health and the environment, proper safety protocols and regulations must be followed during the entire mining and extraction process to protect workers and the surrounding ecosystem.
Processing and Refining of Cadmium Ore

Processing and refining of cadmium ore involve several steps to extract cadmium in its pure form. As mentioned earlier, cadmium is typically obtained as a byproduct during the extraction of zinc, lead, or copper ores. Here’s an overview of the typical processing and refining process for cadmium:
- Ore Preparation: Cadmium ore is first crushed and ground into a fine powder to increase its surface area for subsequent processing. This step allows for the efficient release of the valuable minerals, including cadmium.
- Flotation: The crushed and ground ore undergoes a process called flotation. In this process, chemicals and reagents are added to the ore slurry to create conditions where valuable minerals (such as zinc, lead, and copper sulfides) are selectively separated from other non-valuable minerals.
- Concentration: The flotation process results in a concentrate that contains various metals, including cadmium. The concentrate is further processed to increase the cadmium content and remove impurities.
- Roasting: The concentrate may undergo roasting, a high-temperature process where it is heated in the presence of air or oxygen. Roasting converts cadmium sulfide minerals (like greenockite) into cadmium oxide (CdO).
- Reduction: The roasted concentrate, containing cadmium oxide, is mixed with carbon (usually in the form of coke) and heated in a furnace. The carbon acts as a reducing agent, reacting with the cadmium oxide to produce metallic cadmium vapor.
- Condensation and Collection: The cadmium vapor produced during the reduction process is then cooled and condensed into a solid form. This condensed cadmium is collected and further processed.
- Refining: The obtained cadmium metal may undergo further refining to remove any remaining impurities and ensure its purity. Several refining techniques may be used, such as:
- Electrolytic Refining: Electrolysis is employed to purify the cadmium metal further. The cadmium is dissolved in a suitable electrolyte, and an electric current is passed through the solution, causing the cadmium ions to migrate to the cathode and deposit pure cadmium.
- Zone Refining: Zone refining is another method used to refine cadmium. In this technique, a heated zone moves through the cadmium, causing impurities to migrate towards the end of the sample, where they are removed, leaving behind a purified cadmium sample.
- Final Product: The end result of the processing and refining of cadmium ore is high-purity cadmium metal, ready for use in various industrial applications.
Throughout the entire processing and refining process, strict safety protocols and environmental regulations must be followed to ensure the safety of workers, protect the environment, and manage potential hazards associated with cadmium and its compounds.
Cadmium Ore Reserves and Production
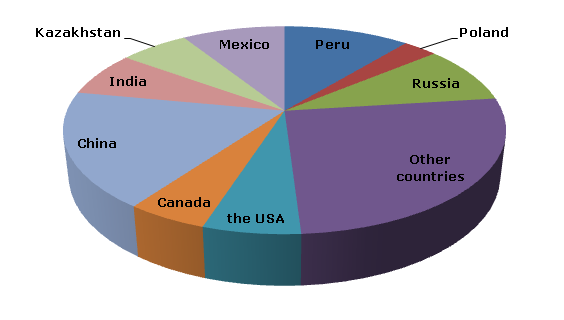
Cadmium is not typically mined as a primary metal but is obtained as a byproduct during the extraction and processing of zinc, lead, and copper ores. As a result, cadmium ore reserves are often associated with the reserves of these base metals.
Cadmium ore reserves and production are influenced by various factors, including demand for zinc, lead, and copper, technological advancements, mining economics, and environmental regulations. The availability of cadmium can vary from year to year based on these factors and global market conditions.
China, Australia, Canada, Peru, and the United States have historically been some of the significant producers of zinc, lead, and copper ores, and thus, they also contribute to the global cadmium production. Additionally, some other countries may have smaller cadmium production as a byproduct of their metal mining activities.
It’s important to note that cadmium production is subject to strict regulations due to its toxic nature, and measures are taken to ensure safe handling, disposal, and environmental protection.
Industrial Uses of Cadmium


Cadmium, despite its toxic nature, has found various industrial applications due to its unique properties. However, it’s important to note that many of these applications have decreased or shifted to alternatives over time due to the health and environmental concerns associated with cadmium. Here are some of the historical and current industrial uses of cadmium:
- Batteries: Historically, cadmium was widely used in rechargeable nickel-cadmium (NiCd) batteries. NiCd batteries were commonly used in portable electronic devices, such as cameras, cell phones, and laptops, due to their high energy density and ability to be recharged. However, in recent years, the use of NiCd batteries has declined due to environmental concerns, and they have been largely replaced by other battery technologies like lithium-ion batteries.
- Electroplating: Cadmium has excellent corrosion resistance, making it suitable for electroplating applications. It is used as a protective coating for various metals, such as steel, to prevent corrosion and improve the appearance of the surface. However, cadmium electroplating is now less common due to environmental and health concerns.
- Pigments: In the past, cadmium compounds were used as pigments in paints, coatings, and plastics to produce bright yellow, orange, and red colors. However, the use of cadmium-based pigments has decreased significantly due to their toxicity, and they have been largely replaced by alternative, non-toxic pigments.
- Alloys: Cadmium can be alloyed with other metals to improve their properties. For example, cadmium is used as a component in some low-melting-point alloys, such as solder and fusible alloys.
- Semiconductors: Cadmium sulfide (CdS) is a semiconductor material that has been used in certain optoelectronic devices, such as photovoltaic cells (solar cells), light sensors, and photocells. However, alternative semiconductor materials are now more commonly used for these applications.
- Stabilizers and Additives: Cadmium compounds have been used as stabilizers and additives in plastics and certain industrial processes. However, their use has declined due to health and environmental concerns.
It’s important to reiterate that many of these applications have faced increasing scrutiny due to the toxic nature of cadmium. In response, there have been efforts to reduce or eliminate cadmium usage in various industries and replace it with safer alternatives. These efforts aim to protect human health, prevent environmental contamination, and promote sustainable practices.
Summary of key points
- Cadmium: Cadmium is a soft, bluish-white metal with the chemical symbol “Cd” and atomic number 48. It is a transition metal and is relatively rare in the Earth’s crust.
- Occurrence: Cadmium is commonly found as an impurity in zinc, lead, and copper ores, rather than occurring as a primary mineral. The main sources of cadmium are as a byproduct during the extraction and refining of these base metals.
- Industrial Uses: Cadmium has historically been used in various industrial applications, including batteries (nickel-cadmium batteries), electroplating, and pigments for paints. However, many of these uses have declined due to environmental and health concerns.
- Toxicity: Cadmium and its compounds are highly toxic to humans and other living organisms. Prolonged exposure to cadmium can cause serious health issues, particularly affecting the kidneys and bones. Proper handling and disposal of cadmium-containing materials are crucial to prevent its release into the environment.
- Environmental Impact: Improper disposal of cadmium-containing waste and industrial emissions can lead to cadmium pollution in soil, water bodies, and the food chain, impacting ecosystems and human health.
- Regulations: Many countries have implemented strict regulations on the use and disposal of cadmium and cadmium-containing products to protect human health and the environment.
- Byproduct: Cadmium is mainly obtained as a valuable byproduct during the extraction and processing of zinc, lead, and copper ores.
- Shift to Alternatives: Due to its toxicity, many industries have sought alternatives to cadmium-based products, such as replacing nickel-cadmium batteries with lithium-ion batteries and using non-toxic pigments in paints.
- Mining and Refining: Cadmium ore is typically extracted alongside zinc, lead, or copper ores. The process involves ore preparation, flotation, concentration, roasting, reduction, condensation, and refining to obtain high-purity cadmium metal.
- Safety and Sustainability: Responsible mining, processing, and handling of cadmium are essential to ensure the safety of workers, protect the environment, and manage potential hazards associated with cadmium and its compounds.
Overall, understanding the properties, sources, uses, and risks associated with cadmium is crucial for adopting responsible practices and protecting human health and the environment from its harmful effects.




































